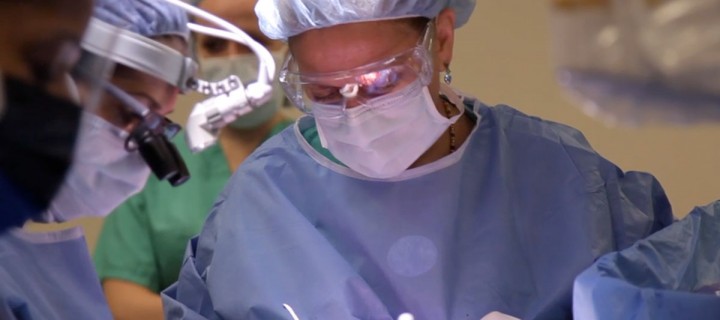
Την πρώτη κλινική δοκιμή θεραπείας με νανοσωματίδια σε ανθρώπους που πάσχουν από μεταστατικό μελάνωμα, πραγματοποίησε ομάδα επιστημόνων στο Memorial Sloan Kettering Cancer Center.
Διαβάστε τη συνέντευξη που δίνει ο καθηγητής, ειδικός σε θέματα νανοθεραπείας Michelle Bradbury .
New Imaging Method Lights Up Cancer Cells during Surgery
Surgery is the first treatment option for most cancer cases, and surgical procedures continue to grow more effective with the adoption of new technologies. One of the biggest remaining challenges, however, is making sure no stray cancer cells are left behind that could form another tumor.
Now a new optical-imaging approach could enable surgeons to more precisely visualize cancerous tissue during an operation. The noninvasive technique combines the use of silica nanoparticles acting as fluorescent probes with a handheld camera that detects light emitted by the particles, creating a high-resolution image showing cancer-cell location. This new method is in the early testing phase with patients.
Memorial Sloan Kettering clinician-scientist Michelle Bradbury, an associate professor of radiology who specializes in nanomedicine, molecular imaging, and neuroradiology, has worked with Ulrich Wiesner, a Cornell University professor of materials science and engineering, to put this nanoparticle–device combination into use in the clinic. She recently received Investigational New Drug approval from the US Food and Drug Administration for the use of these particles in humans — the first such approval for particles of this type. We asked Dr. Bradbury to give an overview of the new technology and explain how it can improve cancer detection and treatment.
What nanoparticles are used in this approach, and how do they work?
Initially developed by Dr. Wiesner, the nanoparticles, called C dots, consist of silica shells encapsulating molecules of dye that glow brightly when hit by light of a specific wavelength. This allows us to trace their location as they move throughout the body. The silica shell actually enhances the brightness and stability of the particles, significantly improving our ability to detect and penetrate cancerous tissues. The nanoparticles are less than one-thousandth the diameter of a red blood cell — small enough to be transported in the blood across tissue to reach a tumor and then be excreted through the kidneys, avoiding toxic buildup in the body. But they are also large enough to stay in the target area for at least 24 hours, unlike many other molecules, which can rush in and out of a tumor site too quickly.
We have further customized the C dots so they can be loaded with more than one substance to perform multiple tasks simultaneously, such as identifying the location of cancer cells using optical and/or positron emission tomography (PET) imaging and potentially delivering drugs. My laboratory extensively evaluated these modified C dots in living systems in preparation for clinical use.
What causes the C dots to go to cancer cells and remain there rather than dispersing throughout the body?
We have tagged the nanoparticles with a molecule called cRGD, which binds to a receptor abundant on the surface of many tumor cells and the cells lining the walls of blood vessels that nourish them. When the C dots encounter these cancer cells, they latch onto the receptor and accumulate within the cells. We are trying to push the optical properties and brightness of the particles to the point that we could detect even a single cancer cell. This will allow us to spot cancer cells at the tumor border as well as cells that have spread from a primary tumor to nearby lymph nodes or to other sites. In this way, we can selectively remove only those nodes that contain cancer cells, which is not possible at present. Beyond surgery, the properties of these C dot imaging probes could allow us to confirm that drugs they may be carrying have actually reached their target.
How does the camera come into play, and what is novel about the technology?
During an operation, and after injection of the nanoparticle, the surgeon focuses the camera on the tumor area. The camera shoots a signal to excite the dye molecules within the nanoparticles, causing the emission of light that is picked up by the camera and transmitted onto a screen. The resolution and detection sensitivity is very high and still improving. Surgeons using the camera can look at the screen and see the cancer cells glowing brightly amid the surrounding tissue, allowing them to be more certain they are removing all malignant cells.
Because the camera is handheld, it’s very flexible. You can go within a few millimeters of the tissue and image areas of anatomy that you could never reach by any other method. In addition, the camera can detect multiple signals at once, so you could inject two different dye-containing particles that could be visualized during the operation — for example, one to mark cancer cells for removal and one to mark cells of critical adjacent structures, such as nerves, to avoid risk of injury.
How is the camera currently being used at Memorial Sloan Kettering?
We are about to test whether these optical technologies work better than conventional radiotracer methods at detecting cancer spread to lymph nodes in melanoma patients. I am leading a study, along with head and neck cancer surgeon Snehal Patel, of patients with head and neck melanomas requiring surgical removal. Before the operation, Dr. Patel injects the standard radioactive tracer and obtains a signal from this tracer when it reaches lymph nodes draining the primary tumor site. After the surgery begins, he also injects C dots around the primary tumor and uses the camera intraoperatively to obtain an optical image. This allows us to compare whether the C dots work better than conventional methods at identifying only cancerous nodes. For any cancer, you don’t want to take out more nodes than necessary — which can cause pain and swelling — but you also want to ensure that every cancer cell is removed.
What does this technology hold for the future?
It opens the door to a wide range of image-guided surgeries that we want to perform here at Memorial Sloan Kettering, using particles as diagnostic and/or therapeutic tools. My collaboration with Dr. Wiesner is critical for expanding the particle and camera technologies in these directions. At first these technologies will be used in open surgical and minimally invasive laparoscopic procedures, but down the road they will be incorporated into robotic systems, leading to image-guided surgeries that are even more precise. Memorial Sloan Kettering surgeons are absolutely crucial to developing these new approaches because they know what is actually practical and useful to them and can keep us constantly informed as we refine these techniques. Also, the versatility of the particles will enable us to adapt them for targeting other tumor types in surgical settings. We feel that in the future, this technology has the potential to change surgical care as we know it, while ensuring better patient outcomes.
The camera used in this new technique is called Artemis Imaging Technology, manufactured by Netherlands-based Quest Medical Imaging. The final device design was developed during a three-year partnership between Quest Medical Imaging and Memorial Sloan Kettering to be used in both conventional open surgery and minimally invasive laparoscopic procedures. These enhancements were further refined to expand the camera’s intraoperative applications through a collaboration between Memorial Sloan Kettering and Cornell University.